Spain leads clinical trials in Europe
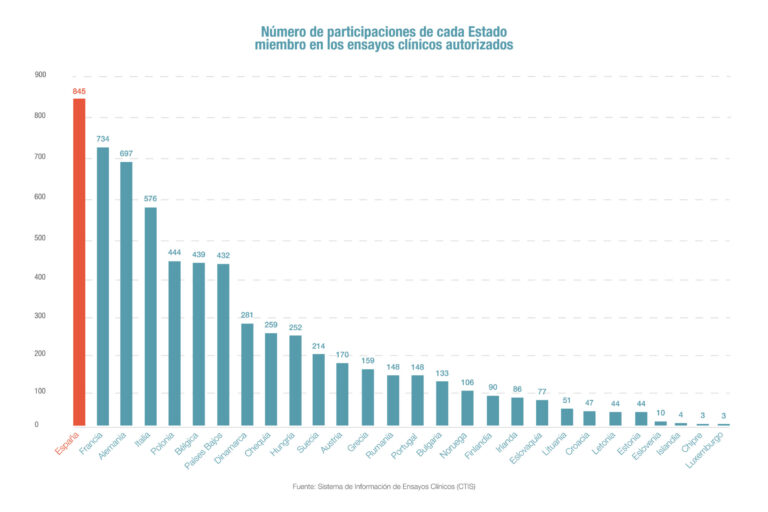
According to Farmaindustria, Spain is the European country with the highest participation in clinical trials. Of the 1,944 clinical trials authorized in the European Union through the new European Information System (CTIS), Spanish sites participated in 845 (43%).…